项目数量-432
医用耗材核酸检测
北检院检测中心 | 完成测试:次 | 2025-05-17
注意:因业务调整,暂不接受个人委托测试望见谅。
检测项目
医用耗材核酸检测体系包含六大核心验证模块:
核酸吸附效率测试:量化评估耗材表面对目标核酸的非特异性吸附率
生物相容性验证:通过细胞毒性试验与致敏试验确认材料安全性
无菌性能检测:依据药典方法进行微生物限度与灭菌效果验证
内毒素测定:采用鲎试剂法检测细菌内毒素残留量
化学残留分析:运用GC-MS检测塑化剂、有机溶剂等化学物质残留
检测范围
本检测体系覆盖三类主要耗材类型:
采样类耗材:包括鼻咽拭子、唾液采集器等与人体直接接触的器械组件
保存运输类耗材:病毒保存液、低温运输管等涉及生物样本稳定性的容器系统
实验处理类耗材:PCR反应管、磁珠纯化板等直接影响检测结果的实验器具
辅助类器材:移液器吸头、离心管等基础实验耗材的质量控制
检测方法
检测仪器
实时荧光定量PCR系统(ABI7500)
-分辨率:0.01拷贝/μL
-温控精度:0.1℃
-符合CNAS-CL01-A025认可要求
全自动酶标仪(BioTekSynergyH1)
-波长范围:200-999nm
-光程校正技术
-符合21CFRPart11规范要求
三重四极杆质谱仪(Agilent6495C)
-质量范围:5-3000m/z
-扫描速度:12500Da/s
-检出限达ppt级别
-通过ISO/IEC17025认证校准程序维护校准状态。
生物安全柜(ESCOAirstream)
-H14级高效过滤器
-风速稳定性5%
-符合NSF/ANSI49标准
-定期进行气流模式验证与粒子计数测试。
激光共聚焦显微镜(LeicaTCSSP8)
-XYZ分辨率:120nm/120nm/400nm
-HyD混合探测器
-活细胞成像系统
-定期执行NIST可追溯性校准。
ISO13485:2016医疗器械质量管理体系标准实施指南
国家药监局《医疗器械生物学评价指导原则》
AAMITIR42:2016核酸相关医疗器械评价规范
CNAS-CL02-A009医学实验室质量和能力认可准则
SAC/TC136全国医用临床检验实验室和体外诊断系统标准化技术委员会文件汇编
AACC临床实验室标准化研究院(CLSI)EP系列文件
TheUnitedStatesPharmacopeia(USP)GeneralChapters
TheInternationalCouncilforHarmonisation(ICH)Q2(R2)Guideline
TheEuropeanPharmacopoeia(Ph.Eur.)BiologicalTests
TheJapanesePharmacopoeia(JP)GeneralTests
TheBritishPharmacopoeia(BP)Appendices
TheInternationalOrganizationforStandardization(ISO)TechnicalReports
TheAmericanSocietyforTestingandMaterials(ASTM)Standards
TheClinicalandLaboratoryStandardsInstitute(CLSI)Guidelines
TheCollegeofAmericanPathologists(CAP)Checklists
TheInternationalFederationofClinicalChemistry(IFCC)Recommendations
TheWorldHealthOrganization(WHO)TechnicalReportSeries
TheEuropeanDirectoratefortheQualityofMedicines&HealthCare(EDQM)Guidelines
TheNationalInstituteofStandardsandTechnology(NIST)ReferenceMaterials
TheFoodandDrugAdministration(FDA)GuidanceDocuments
TheEuropeanMedicinesAgency(EMA)ScientificGuidelines
ThePharmaceuticalsandMedicalDevicesAgency(PMDA)Standards
TheTherapeuticGoodsAdministration(TGA)RegulatoryRequirements
TheHealthSciencesAuthority(HSA)TechnicalGuidance
TheMedicalDeviceCoordinationGroup(MDCG)PositionPapers
TheGlobalHarmonizationTaskForce(GHTF)StudyGroupsDocuments
TheInternationalMedicalDeviceRegulatorsForum(IMDRF)Reports
TheAsianHarmonizationWorkingParty(AHWP)TechnicalDocuments
ThePanAmericanHealthOrganization(PAHO)TechnicalNotes
TheAfricanMedicinesRegulatoryHarmonization(AMRH)InitiativePublications
TheGulfCooperationCouncil(GCC)RegulatoryGuidelines
TheASEANMedicalDeviceDirective(AMDD)ImplementationFramework
TheEurasianEconomicUnion(EAEU)TechnicalRegulations
TheSouthernAfricanDevelopmentCommunity(SADC)HarmonizedRequirements
TheEastAfricanCommunity(EAC)MedicalDeviceRegulations
TheWestAfricanHealthOrganization(WAHO)StandardsCompendium
TheAsia-PacificEconomicCooperation(APEC)RegulatoryCooperationPlan
TheOrganisationforEconomicCo-operationandDevelopment(OECD)ConsensusDocuments
TheInternationalAccreditationForum(IAF)MandatoryDocuments
TheInternationalLaboratoryAccreditationCooperation(ILAC)Guidelines
TheJointCommissionInternational(JCI)AccreditationStandards
TheNationalAccreditationBoardforTestingandCalibrationLaboratories(NABL)Criteria
TheChinaNationalAccreditationServiceforConformityAssessment(CNAS)Rules
TheAmericanAssociationforLaboratoryAccreditation(A2LA)Requirements
TheUnitedKingdomAccreditationService(UKAS)TechnicalBulletins
TheDeutscheAkkreditierungsstelle(DAkkS)Regulations
TheComitFranaisd'Accrditation(COFRAC)Directives
TheJapanAccreditationBoardforConformityAssessment(JAB)Standards
TheKoreaLaboratoryAccreditationScheme(KOLAS)Manuals
TheSingaporeAccreditationCouncil(SAC-SINGLAS)Criteria
TheHongKongAccreditationService(HKAS)GuidanceNotes
TheTaiwanAccreditationFoundation(TAF)DocumentationSystem.
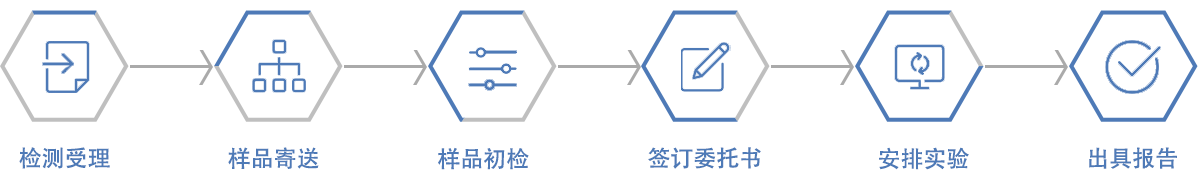
线上咨询或者拨打咨询电话; 获取样品信息和检测项目; 支付检测费用并签署委托书; 开展实验,获取相关数据资料; 出具检测报告。检测流程
上一篇:清灰剂成分检测
下一篇:小学教室灯光采光检测









